
Illustration by Edgar Bąk
It would take a foolhardy physicist to dare attempt to break the laws of thermodynamics. But it turns out that there may be ways to bend them. At a lab at the University of Oxford, UK, quantum physicists are trying to do so with a small lump of synthetic diamond. At first, the diamond is barely visible, nestled inside a chaotic mess of optical fibres and mirrors. But when they switch on a green laser, defects in the diamond are illuminated, and the crystal begins to glow red.
In that light, the team has found preliminary evidence of an effect that was theorized only a few years ago1: a quantum boost that would push the diamond's power output above the level prescribed by classical thermodynamics. If the results hold up, they will be a tangible boon for the study of quantum thermodynamics, a relatively new field that aims to uncover the rules that govern heat and energy flow at the atomic scale.
There is reason to suspect that the laws of thermodynamics, which are based on how large numbers of particles behave, are different in the quantum realm. Over the past five years or so, a quantum-thermodynamics community has grown around that idea. What was once the domain of a handful of theoreticians now includes a few hundred theoretical and experimental physicists around the globe. “The field is moving so fast I can barely keep up,” says Ronnie Kosloff, an early pioneer of the field at the Hebrew University of Jerusalem in Israel.
Related stories
- Battle between quantum and thermodynamic laws heats up
- Quantum gas goes below absolute zero
- The unavoidable cost of computation revealed
A number of quantum thermodynamicists hope to find behaviour outside the remit of conventional thermodynamics that could be adapted for practical purposes, including improving lab-based refrigeration techniques, creating batteries with enhanced capabilities and refining technology for quantum computing.
But the field is still in its infancy. Experiments such as the one taking place at Oxford are just starting to put theoretical predictions to the test. And physicists working at the periphery are watching such tests closely for evidence of the useful applications that theorists have predicted. “Quantum thermodynamics is clearly hot — pardon the pun,” says Ronald Walsworth, a physicist at Harvard University in Cambridge, Massachusetts, who specializes in developing precision atomic-scale tools. “But for those of us looking in from the outside, the question is: can it really shed new light on the development of technologies?”
Breaking the law
The development of the classical laws of thermodynamics stretches back to the nineteenth century. They emerged from the effort to understand steam engines and other macroscopic systems. Thermodynamic quantities such as temperature and heat are statistical in nature and defined in reference to the average motion of large ensembles of particles. But back in the 1980s, Kosloff began pondering whether this picture would continue to make sense for much smaller systems.
It wasn't a popular line of research at the time, says Kosloff, because the questions being asked were largely abstract, with little hope of connection to experiments. “The field developed very slowly,” he says. “I was alone for years.”

Jonas Becker
An experimental set-up at the University of Oxford is used to investigate whether quantum effects can enhance the power output of a diamond.
That changed dramatically around a decade ago, as questions about the limits of technological miniaturization became more pressing and experimental techniques advanced. A flurry of attempts were made to calculate how thermodynamics and quantum theory might combine. But the resulting proposals created more confusion than clarity, Kosloff says. Some claimed that quantum devices could violate classical thermodynamic constraints with impunity and so act as perpetual-motion machines, capable of performing work without needing any energy input. Others, suggesting that the laws of thermodynamics should hold unmodified at very small scales, were equally perplexing. “In some sense, you can use the same equations to work out the performance of a single atom engine and your car engine,” says Kosloff. “But that seems shocking, too — surely as you get smaller and smaller you should hit some quantum limit.” In classical thermodynamics, a single particle doesn't have a temperature. So as both the system generating work and its environment approach that limit, it becomes increasingly absurd to imagine that they would obey standard thermodynamic rules, says Tobias Schaetz, a quantum physicist at the University of Freiburg in Germany.
The preponderance of conflicting theoretical claims and predictions initially undermined the burgeoning field's credibility. “I have been very critical of the field because there is far too much theory and not enough experiment,” says quantum physicist Peter Hänggi, at the University of Augsburg in Germany. But the community is beginning to coalesce more formally around core questions in an effort to cut through the chaos. One goal has been to use experiments to uncover the point at which the classical laws of thermodynamics no longer perfectly predict the thermal behaviour of quantum systems.
Experiments are starting to pin down that quantum–classical boundary. Last year, for example, Schaetz and his colleagues showed that, under certain conditions, strings of five or fewer magnesium ions in a crystal do not reach and remain in thermal equilibrium with their surroundings like larger systems do2. In their test, each ion started in a high-energy state and its spin oscillated between two states corresponding to the direction of its magnetism — 'up' and 'down'. Standard thermodynamics predicts that such spin oscillations should die down as the ions cool by interacting with the other atoms in the crystal around them, just as hot coffee cools when its molecules collide with molecules in the colder surrounding air.
Such collisions transfer energy from the coffee molecules to the air molecules. A similar cooling mechanism is at play in the crystal, where quantized vibrations in the lattice called phonons carry heat away from the oscillating spins. Schaetz and his colleagues found that their small ion systems did stop oscillating, suggesting that they had cooled. But after a few milliseconds, the ions began oscillating vigorously again. This resurgence has a quantum origin, says Schaetz. Rather than dissipating away entirely, the phonons rebounded at the edges of the crystal and returned, in phase, to their source ions, reinstating the original spin oscillations.
Schaetz says that his experiment sends a warning to engineers attempting to reduce the size of existing electronics. “You may have a wire that is only 10 or 15 atoms wide, and you may think that it has successfully carried the heat away from your chip, but then boop — suddenly this quantum revival happens,” Schaetz says. “It is very disturbing.”
Rebounding phonons could present a challenge in some applications, but other quantum phenomena could turn out to be useful. Efforts to identify such phenomena had been stalled by the difficulty in defining basic quantities, such as heat and temperature, in quantum systems. But the solution to a famous thought experiment, laid out 150 years ago by Scottish physicist James Clerk Maxwell, provided a clue about where to turn, posing an intriguing link between information and energy. Maxwell imagined an entity that could sort slow- and fast-moving molecules, creating a temperature difference between two chambers simply by opening and closing a door between them.
Such a 'demon', as it was later called, thus generates a hot and a cold chamber that can be harnessed to produce useful energy. The problem is that by sorting particles in this way, the demon reduces the system's entropy — a measure of the disorder of the particles' arrangements — without having done any work on the particles themselves. This seemingly violates the second law of thermodynamics.
But physicists eventually realized that the demon would pay a thermodynamic price to process the information about the molecules' speeds. It would need to store, erase and rewrite that information in its brain. That process consumes energy and creates an overall increase in entropy3. Information was once thought to be immaterial, “but Maxwell's demon shows that it can have objective physical consequences”, says quantum physicist Arnau Riera, at the Institute of Photonic Sciences in Barcelona, Spain.
Finding the limit
Inspired by the idea that information is a physical quantity — and that it is intimately linked to thermodynamics — researchers have attempted to recast the laws of thermodynamics so that they work in the quantum regime.
Perpetual-motion machines may be impossible. But an early hope was that limits prescribed by quantum thermodynamics might be less stringent than those that hold in the classical realm. “This was the train of thought we had learned from quantum computing — that quantum effects help you beat classical bounds,” says Raam Uzdin, a quantum physicist at the Technion–Israel Institute of Technology in Haifa.
Disappointingly, Uzdin says, this is not the case. Recent analyses suggest that quantum versions of the second law, which governs efficiency, and the third law, which prohibits systems from reaching absolute zero, retain similar and, in some cases, more-stringent constraints than their classical incarnations.
Some differences arise because the macroscopic thermodynamic quantity 'free energy'— the energy a system has available to do work — doesn't have just one counterpart at the microscale, but many, says Jonathan Oppenheim, a quantum physicist at University College London. Classically, the free energy is calculated by assuming that all states of the system, determined by the arrangement of particles at a given energy, are equally likely. But that assumption isn't true on tiny scales, says Oppenheim; certain states might be much more probable than others. To account for this, additional free energies need to be defined in order to accurately describe the system and how it will evolve. Oppenheim and his colleagues propose that individual second laws exist for each type of free energy, and that quantum devices must obey all of them4. “Since the second law tells you what you aren't allowed to do, in some ways, it seems that having more laws on the microscale leaves you worse off,” says Oppenheim.
“The field is moving so fast I can barely keep up.”
Much of the work done to calculate equivalents of the second and third laws remains, for now, theoretical. But proponents argue that it can help to illuminate how thermodynamic bounds are physically enforced at small scales. For instance, a theoretical analysis carried out by a pair of quantum physicists based in Argentina showed that as a quantum refrigerator nears absolute zero, photons will spontaneously appear in the vicinity of the device5. “This dumps energy into the surroundings, causing a heating effect that counters the cooling and stops you ever reaching absolute zero,” explains team member Nahuel Freitas of Ciudad University in Buenos Aires.
Theory has also revealed some potential wiggle room. In a theoretical analysis examining information flow between hot and cold chambers, or 'baths', of particles, a team based in Barcelona that included Riera and quantum physicist Manabendra Nath Bera discovered a strange scenario in which the hot bath seemed to spontaneously get hotter, while the cold bath became colder6. “At first, this looks crazy, like we can violate thermodynamics,” says Bera. But the researchers soon realized that they had overlooked the quantum twist: the particles in the baths can become entangled. In theory, making and breaking these correlations provides a way to store and release energy. Once this quantum resource was budgeted for, the laws of thermodynamics fell into place.
A number of independent groups have proposed using such entanglement to store energy in a 'quantum battery', and a group at the Italian Institute of Technology in Genoa is attempting to confirm the Barcelona team's predictions with batteries built from superconducting quantum bits, or 'qubits'7. In principle, such quantum batteries could charge considerably faster than their classical equivalents. “You won't be able to extract and store more energy than the classical bound allows — that's set by the second law,” says Riera. “But you may be able to speed things up.”
Some researchers are looking for easier ways to manipulate qubits for quantum-computing applications. Quantum physicist Nayeli Azucena Rodríguez Briones at the University of Waterloo in Canada and her colleagues have devised8 an operation that might enhance the cooling needed for quantum-computing operations by manipulating pairs of qubit energy levels. They are currently planning to test this idea in the lab using superconducting qubits.
A small spark
The concept that quantum effects could be exploited to improve thermodynamic performance also inspired the diamond experiment under way at Oxford, which was first proposed by Kosloff, Uzdin and Amikam Levy, also at the Hebrew University1. Defects created by nitrogen atoms scattered through the diamond can serve as an engine — a machine that performs an operation after being brought into contact with first a hot reservoir (in this case a laser) and then a cold one. But Kosloff and his colleagues expect that such an engine can be operated in an enhanced mode, by exploiting a quantum effect that enables some of the electrons to exist in two energy states simultaneously. Maintaining these superpositions by pulsing the laser light rather than using a continuous beam should enable the crystal to emit microwave photons more rapidly than it otherwise would (see 'Building a quantum heat engine').
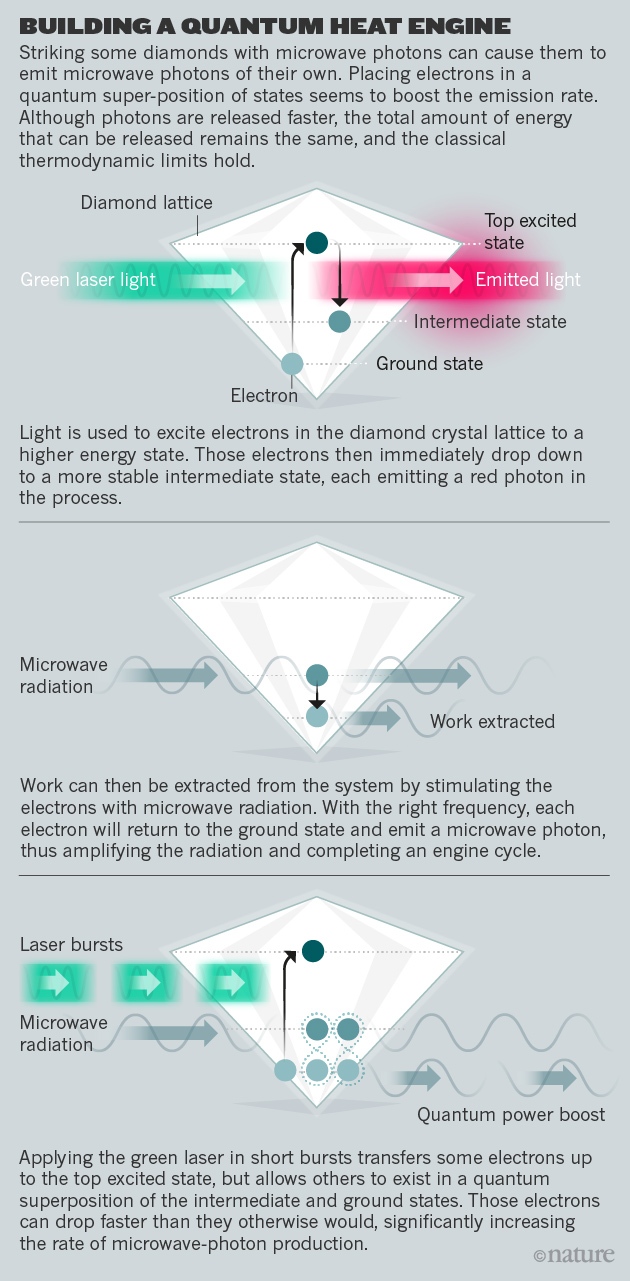
Last week, the Oxford-based team posted a preliminary analysis9 showing evidence of the predicted quantum boost. The paper has yet to be peer reviewed, but if the work holds up, then “it is a groundbreaking result,” says Janet Anders, a quantum physicist at Exeter University, UK. But, she adds, it's still not clear exactly what enables this feat. “It seems to be a magic fuel, not so much adding energy, but enabling the engine to extract energy faster,” Anders says. “Theoretical physicists will need to examine just how it does this.”
Focusing on experiments is a major step in the right direction for revitalizing the field, says Hänggi. But, for him, the experiments are not yet bold enough to give truly ground-breaking insights. There is also the challenge that quantum systems can be irrevocably disturbed by measurement and interaction with the environment. These effects are rarely sufficiently accounted for in theoretical proposals for new experiments, he says. “That is difficult to calculate, and much more difficult to implement in an experiment,” he says.
Ian Walmsley, who heads the Oxford lab where the diamond experiment was conducted, is also circumspect about the future of the field. Although he and other experimenters have been drawn to quantum thermodynamics research in recent years, he says that their interest has been largely “opportunistic”. They have spotted the chance to carry out relatively quick and easy experiments by piggybacking on set-ups already in place for other uses; the diamond-defect set-up, for instance, is already being widely studied for quantum computing and sensor applications. Today, quantum thermodynamics is fizzing with energy, Walmsley says. “But whether it will continue to sparkle, or just explode into nothing, well, we will have to wait and see.”
- Journal name:
- Nature
- Volume:
- 551,
- Pages:
- 20–22
- Date published:
- ()
- DOI:
- doi:10.1038/551020a
